 Figure 1
Figure 1
Dissect the head away from the body. With a number 11 scalpel blade, gently cut away one of the eyes to expose the inside of the head. Avoid putting pressure on the head when cutting (making the other eye 'bulge'). Place the dissected head into 2% glutaraldehyde in phosphate buffer. This solution should be on ice. Often it is necessary to tap the head down into the solution as it has a tendency to float. Alternatively, you can give them a 5 second spin in a microfuge to submerge it.
Add an equal volume of 2% OsO4 in phosphate buffer. Only handle OsO4 in the hood and wear gloves; it is volatile and extremely toxic. Incubate the mixture for 30 min on ice. Remove the glutaraldehyde/OsO4 mixture and wash the tissue with cold phosphate buffer by filling the tube completely. Dispose of waste glutaraldehyde and OsO4 by adding an excess of dry milk powder to the waste solutions in a bottle. The deactivated solution can then be safely removed. Add fresh OsO4 to the tissue and incubate on ice for 1-2 hours. Discard the OsO4 and dehydrate the tissue by successive 10 min incubations on ice in the following ethanol solutions: 30%, 50%, 70%, 90%, 100%. For preparing tissue for electron microscopic analysis, also include an 80% EtOH wash. Repeat the final 100% EtOH incubation.
Replace the final alcohol treatment with propylene oxide and incubate for 10 min at room temperature. Handle propylene oxide with gloves and only use it in the hood. Repeat the propylene oxide wash twice. Add an equal volume of durcapan resin to the last propylene oxide wash and thoroughly mix. Handle resin with non-latex gloves as it is carcinogenic when unpolymerized. Incubate overnight at room temperature. Use soft resin for semi-thin and thick sectioning and use hard resin for thin sectioning.
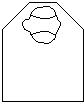
Replace the resin/propylene oxide mixture with pure resin and incubate at least 4 hours. Place tissue in a drop of resin on a slide. Examine the head under a dissecting microscope and discard any poorly fixed heads. Dissect away unwanted tissue from the head. Using a needle, transfer a fixed head to a mold which has been filled with resin. Useful molds are BEEM or silicone rubber flat embedding molds (Ted Pella Co.). Place the head very close to the edge of the mold (Figure 1). Orient the head so that the head is anterior up and the eye to be sectioned is closest to the edge. Make sure that the head is flat and resting on the bottom of the mold. Bake the resin at 70C overnight. Bake any waste resin before discarding.
 Figure 1
Figure 1
Examine the embedded heads to ensure that they did not shift position while the resin hardened. Only section those which maintained their correct orientation. Trim the resin block with a razor blade, preferably the kind which are Teflon coated. Trim away excess resin which covers the eye. This is diagrammed in Figure 2. Start by trimming resin to form the face of the block which will be cut. Then cut away resin to form a trapezoid shape, so that the final form resembles a pyramid with its top lopped off.

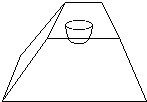 Figure 2
Figure 2
Cut sections with your favorite microtome. Section thickness can range from 0.5 to 5 microns for tissue embedded in soft resin, and from 50 to 300 nm for tissue embedded in hard resin. Sections can be collected either with a wire loop or a shaved wooden stick. The stick is best for collecting serial sections when section order must be preserved.
Transfer the sections to a puddle of water on a subbed slide. Place the slide on a heating block (about 80C) and allow the water to evaporate. If you wish to examine unstained sections, add a drop of DPX (Fluka) and a cover slip. If you want to stain the eye sections, use toluidine blue. Place slides with sections on them onto a heating block which is at a temperature of 70-80C. Allow the slides to warm up. Add several drops of toluidine blue solution (enough to only cover the sections) to the slide, and incubate. The incubation time is variable: it depends on the temperature of the block, how much toluidine solution is added, etc. Therefore, make an empirical test by staining for several different amounts of time. Around 30 seconds is usually optimal. After incubation, remove the slide and rinse thoroughly with water Examine under the microscope for how well the staining worked. If necessary, you can repeat the procedure until staining is optimal. Take care; it is possible to overstain tissue with this procedure. Allow the slides to completely dry before mounting in DPX.
SOFT HARD (grams) (grams) Resin A 54 50 Hardener B 44.5 50 Accelerator C 2.5 1.75 Plasticiser D 10 0.75
original text by Richard Carthew