Integration and Diversity of Signaling Pathways
Activated by the GHRH Receptor
Genetic studies in mice reveal that GHRH plays an important role in
stimulating somatotroph cell proliferation. For example, overexpression
of GHRH in transgenic mice leads somatotroph cell hyperplasia associated
with GH excess and gigantism. Conversely, loss of GHRH signaling in
the little mouse, which harbors a naturally occurring GHRH
receptor mutation, leads to somatotroph cell hypoplasia associated with
GH deficiency and dwarfism. Classical signaling by the GHRH receptor
is through activation of a stimulatory G protein and cAMP-dependent
signaling pathways (Figure 6). To understand the signaling pathways
leading to GHRH-stimulated cell proliferation, we investigated activation
of the mitogen-activated protein kinase pathway in pituitary cells.
We found that GHRH strongly stimulates both phosphorylation and activation
of the MAP kinases ERK-1 and ERK-2 in pituitary cells. This is replicated
in heterologous cells expressing the GHRH receptor, and is dose- and
time-dependent. GHRH activation of MAP kinase appears to be cAMP dependent,
but is not blocked by an inhibitor of protein kinase A, the enzyme that
mediates most of the effects of cAMP. We are continuing studies to investigate
the pathway of MAP kinase activation in these cells and its role in
the proliferative response.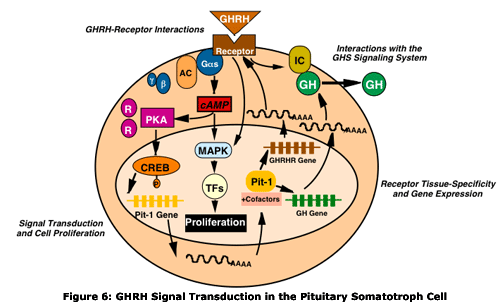
We are also investigating the interaction of the GHRH signaling system with a novel growth hormone regulatory system. The growth hormone secretagogues (GHS) include a family of synthetic compounds that release growth hormone. They act through the G protein-coupled GHS receptor, and a probable endogenous ligand for this receptor, the stomach peptide ghrelin, was recently identified. GHS and ghrelin act through a calcium signaling pathway, in contrast to the cAMP signaling pathway activated by GHRH. GHRH and GHS act synergistically in vitro and in vivo to induce growth hormone secretion, suggesting an intersection of these pathways. We have generated transfected cell populations that express the GHRH receptor, the GHS receptor, or both receptors and shown that they recapitulate the synergism seen in normal pituitary cells. Studies to investigate the mechanistic basis of this synergism suggest a potential interaction between the GHRH and GHS receptors, and this is now being explored in greater detail.
We and others have found that one mechanism by which diversity in GHRH receptor signaling is generated is through the formation of receptor variants that result from alternative RNA processing. In the rat receptor, a major splice variant results from inclusion of an alternative exon, introducing 41 in-frame amino acids into the receptor protein within the third cytoplasmic domain. we found that this variant receptor can be expressed on the cell surface and is normally glycosylated. It binds hormone with an affinity indistinguishable from that of the "normal" receptor, but it is unable to signal though the cAMP pathway. Signaling through other potential pathways is being investigated. Interesting, alternative splicing of the human GHRH receptor at this same exon-intron junction in pituitary adenomas generates a prematurely truncated receptor that appears to have dominant inhibitory actions. We are continuing studies to explore the physiologic significance and regulation of these variant receptor forms.
![]()