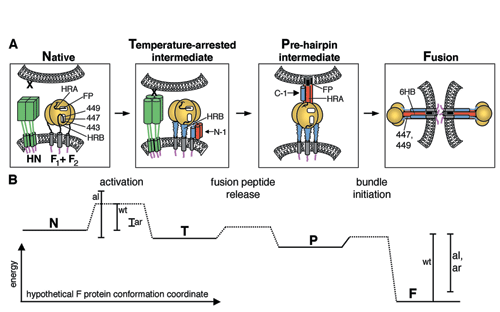

Fig. 1. Model of paramyxovirus-mediated membrane fusion. (A) Our previous studies have shown the paramyxovirus F protein adopts a series of conformations while mediating membrane fusion (Russell et al., 2001; EMBO J. 20 4024-4034): the Native structure (which is in a metastable conformation), a Temperature-arrested intermediate (which forms after HN binds its receptor at non-fusion permissive temperatures and is susceptible to N-1 binding), a Prehairpin intermediate (which adheres to target cells independent of HN and is susceptible to C-1 binding), and the Fusogenic form of F (which couples 6HB formation to membrane merger). The present work is consistent with residues 447 and 449 having distinct interaction sites on the F protein, one in the Native structure and the other in the cavity formed by HRA trimers in the 6HB of the Fusogenic form. The strong correlation between 6HB stability and C-peptide inhibitory potency in the present study and the coincidence of C-1 inhibition and F protein binding in a previous study (Russell et al., 2001) show that C-1 inhibits membrane fusion by binding to transiently exposed HRA triple-stranded coiled coils in the Pre-Hairpin Intermediate. (B) Biophysical studies on the N-1/C-peptide mutants show that the aliphatic (al) and aromatic (ar) mutations at L447 and I449 decrease the amount of energy released by mutant 6HB formation; whereas, functional studies on the F mutants are consistent with the aromatic (ar) mutations decreasing and the aliphatic (al) mutations increasing the activation energy of the Native state. The hyperactive fusion phenotype of the aromatic mutants is consistent with the facile activation (and subsequent inactivation in the absence of a fusion target) of these F mutants overcompensating for the decrease in energy released by mutant 6HB formation. (From Russell, C.J., Kantor, K.L, Jardetzky, T.S. and Lamb, R.A. (2003). J. Cell Biol. 163 , 363-374.)