| |
| |
|
|
| |
|
|
| Symbol: Hh
|
Flybase ID: {Flybase_ID} |
| Synonyms: {Name}
|
{GadFly} |
| Function: {Short_Function} |
{LocusLink} |
| Keywords: {Keywords} |
{Interactive_Fly} |
{Summary}
|
|
|
- Regulating the growth and patterning of the wing and other appendages
in adult segment polarity
- In the embryo hh maintains wg transcription at the boundary of each
segmental unit
- Involved in the development of:
- wing (Mohler 1988; Basler,
1994; Tabata,
1994)
- leg (Diaz-Benjumea et al.1994)
- eye discs (Heberlein et al.1995; Dominguez 1999)
- germ-cell migration (Deshpande et al.2001)
- optic lamina (Huang and Kunes 1996,1998)
- gonad (Forbes et al.1996;Zhang and Kalderon 2000)
- abdomen (Struhl et al.1997)
- gut (Pankratz and Hoch 1995)
- tracheal system (Glazer and Shilo 2001).
|
|
|
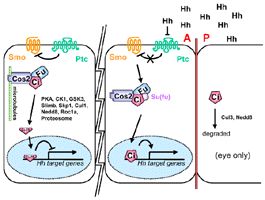
- Ci signaling complex
- Stimulation of cells by Hh releases the complexes (Cos-2, Fu,
Su (fu), Ci) from microtubules and induces the phosphorylation of
both the Fu (Therond,
1996) and Cos-2 (Robbins,
1997) components of the complexes.
- Costal-2
- Engrailed
- Hh induces the expression of en at late stages of wing disc development
(Blair, 1992; Sanchez-Herrero, 1996; Strigini, 1997; Alves, 1998)
- In embryos homozygous for loss-of-function alleles of hh, expression
of en is lost from ectodermal cells after gastrulation (DiNardo,
1988), indicating that the activity of hh, like that of wg, is also
required for the maintenance of en transcription. Although ubiquitous
expression of wg results in the ectopic activation of en (Noordermeer,
1992), the distribution of En protein is unaltered in heat-shocked
HS-hh embryos (Ingham,
1993: Fig 2C). Thus, although necessary for the maintenance
of en, expression of hh is not sufficient for its activation.
- Fused
- Hh induces the phosphorylation of Fu (Therond,
1996)
- in a fu mutant there is no expression of wg in the
ventral ectodermal cells of each parasegment in the embryo, though
transcript is still found in other cells where wg expression
is independent of hh function. The phenotype stays the same
even if Hh is overexpressed with HS-hh. (Ingham,
1993)
- transcription of hh persists for longer in embryos lacking
wild-type fu activity (Fig 2a), suggesting that fu may act 'downstream'
of hh to regulate wg transcription (Ingham,
1993)
- Wingless
- in a fu mutant there is no expression of wg in the
ventral ectodermal cells of each parasegment in the embryo, though
transcript is still found in other cells where wg expression
is independent of hh function. The phenotype stays the same
even if Hh is overexpressed with HS-hh. (Ingham,
1993)
- In en-Gal4/UAS-wg; hh- embryos Winglessis
spreads posterior to the engrailed domain as if a barrier had been
lifted or Wingless movement enhanced (Figure 5B). Wingless protein
distribution is symmetrical, and this is reflected in the cuticle
pattern: in contrast to en-Gal4/UAS-wg embryos, en-Gal4/UAS-wg;
hh- embryos lack rows 2–4 and, instead, have
an extra expanse of naked cuticle (Figure 5C). At the positions
where rows 5 and 6 normally form, lies a thin stripe of small denticles.
Naked cuticle is specified equally in the anterior and posterior
directions, as shown by marking the winglessexpressing cells with
GFP (Figure 5C). Thus, in the absence of hedgehog, wingless action
is symmetric. (Sanson,
1999)
- This effect is due to Hedgehog signaling since the cuticle
phenotype of en-Gal4/UAS-wg; ci- embryos is
identical to that of en-Gal4/UAS-wg; hh- embryos
(Figure 5G). This requirement is dose sensitive, since in hedgehog
or cubitus interruptus heterozygotes, Wingless produced
in the engrailed domain generates occasional breaches of naked
cuticle in the denticle belts (Figures 5H and 5K ). (Sanson,
1999)
- Changes in transcriptional activity that are eleicited by Hh signaling
require Ci (Forbes,
1993)
- Hh signaling reduces phosphorylation of Ci155, which appears to decrease
processing in cultured cell line (Chen,
1999)
- In the ventral ectoderm, Hh signals in both directions leading to
the expression of the patched (ptc) gene in all neighboring cells (Nakano
et al., 1989; Hooper and Scott, 1989; Ingham,,
1991).
- In the ectoderm two observations suggest that anterior and posterior
cells might have different abilities to respond to Hh: (1) only the
anterior neighbouring cells express wg in a Hh-dependent manner; (2)
Hh patterns only those cells posterior to its expression domain in the
dorsal ectoderm (Heemskerk,
1994)
- In smo clones (no Hh signaling) dpp-lacZ, Ptc and anterior En expression
is inhibited, suggesting that they are direct targets for regulation
by Hh signaling (Strigini,
1997)
- Hh patterns the vein 3-4 region (Strigini,
1997)
- A Ptc-related protein, Dispatched (Disp), is specifically required
for the controlled release of Hh-Np (Burke,
1999).
- In the absence of Disp function, Hh-expressing cells accumulate
high levels of Hh but fail to secrete it; as a consequence, Hh target
genes are not activated in responding cells. This requirement is
completely overridden when Hh-Nu is expressed in disp mutant cells
(Burke,
1999), indicating that it is only needed for the secretion of
the lipid-modified form of Hh-N
- Disp is required in the embryonic ectoderm as well as in the posterior
compartment of the imaginal disc (Burke,
1999), implying that the release of Hh- Np is essential for
both the long-range and short-range modes of Hh signaling
- Reduction of the hh dosage (hhts2) enhanced
the partial fusion of L3 and L4 observed in kn1/col1
wings (Vervoort,
1999)
- The anterior displacement, partial loss of L3 and increased width
of the L3–L4 intervein that are observed upon overexpression of
hh in its own domain (UAS-hh/engrailed (en)-Gal4 driver,(Figure 1e)
were suppressed by reducing col dosage (Figure 1f).(Vervoort,
1999)
- The expression of col in the L3–L4 intervein was completely lost
in hhts2 discs raised to 30°C (null condition); it was also lost,
or severely reduced, at 25°C (Figure 4b) (Vervoort,
1999), in contrast to dpp, the expression of which is lost at 30°C
but is normal at 25°C (Strigini,
1997). This indicates that col activation requires Hh activity,
and that it requires Hh levels superior to those required for activation
of dpp.
|
|
|
- Appears as if hh is sequestered by Ptc
|
Transcriptional
Regulation |
- Regulation of Hh transcription:
- Initial transcription of hh in posterior cells (in embryos) is
not dependent on en (Lee, 1992; Mohler, 1992; Tabata, 1992; Tashiro,
1993)
- In imaginal wing discs en activity is both necessary and sufficient
to drive hh expression in a cell autonomous manner (Burke,
1999)
- Mutant clones in the posterior compartment lacking both en and
invected have a loss of hh expression (Sanicola, 1995)
- Hh regulates other proteins:
- Hh binding causes removal of Ptc from surface (Denef,
2000)
- Hh causes phosphorylation, stabilization, and accumulation of
Smo at cell surface [comparable effects when Ptc is removed by RNAi]
(Denef,
2000)
|
|
|
| {Structure} |
Location (protein
and transcript) |
- Hh-Np is tethered to the membrane but is released through the fxn
of Disp (Burke,
1999)
- In the embryonic ectoderm Hh has been shown to accumulate preferentially
basolaterally (Taylor et al. 1993; Tabata,
1994).
|
Protein
Modifications and Regulation |
- Hh protein processing:
- Hh family proteins are synthesized as ~45-kD precursor proteins
that undergo an intramolecular cleavage (Lee,
1994; Bumcrot et al. 1995) that is catalyzed by the C-terminal
portion of the precursor (Lee,
1994; Porter et al. 1995).This reaction yields a 25-kD C-terminal
fragment that has no other known function and an ~19- kD N-terminal
fragment (referred to as Hh-N) that is sufficient for all known
Hh signaling activity.
- This autocleavage of Hh proceeds via a thioester intermediate
that undergoes a nucleophilic attack by cholesterol, resulting in
the covalent coupling of cholesterol to the C terminus of Hh-N to
yield the processed form of the signaling moiety, denoted Hh-Np
("p" standing for processed) (Porter et al.1996b).
- cells expressing an unmodified form of Hh-N (generated by
a C-terminally truncated form of the coding region, which circumvents
the autoproteolysis and hence the cholesterol coupling step)
secrete large quantities of this unmodified form, Hh-Nu, into
the medium (Bumcrot et al. 1995; Porter et al. 1995). In line
with these findings, expression of the Hh-Nu in the embryonic
ectoderm was found to have effects consistent with an increased
range of Hh activity (Porter et al. 1996a). Little Hh-Np is
found in medium conditioned by cells expressing the full-length
protein (Pepinsky et al. 1998; Zeng et al. 2001).
- One interpretation of these results is that Hh-Nu can move
further than Hh-Np due to the absence of the cholesterol modification.
Intriguingly, Ptc contains a sterol-sensing domain (SSD, reviewed
by Osborne and Rosenfeld, 1998), which has been shown in proteins
such as HMG CoA reductase (Gil et al., 1985) and SREBP cleavage-activating
protein (SCAP, Hua et al., 1996) to be able to monitor sterol
levels in membranes. One possibility is that Ptc interacts directly
with the cholesterol moiety of Hh-Np via its SSD, thus sequestering
Hh and restricting its motility (Beachy et al., 1997)
- Such cholesterol-mediated membrane anchoring could thus explain
the restricted range of Hh in the Drosophila embryo and in certain
contexts in vertebrate embryos (such as tooth and hair development),
where Hh appears to act at short range, but it seems at odds
with the long-range signaling activities of the protein in the
limbs and neural tube.
- Shh (Pepinsky et al. 1998), as well as Drosophila Hh (Chamoun
et al. 2001), is palmitoylated on its most N-terminal cysteine.
- The sightless/skinny hedgehog (sig/ski) gene, encodes a polytopic
transmembrane protein with similarity to mammalian acyl transferases
that catalyze O-linked acyl transfers, most likely occuring
through a thioester intermediate(Chamoun et al. 2001).
- Hh-N is rendered inactive in sig/ski mutants (Chamoun
et al. 2001; Lee and Treisman 2001), however an un-acylable
form of Shh retains some activity when expressed in transgenic
Drosophila imaginal discs (Chamoun et al. 2001; Lee and
Treisman 2001).
- In contrast, studies of Shh modification in tissue culture
cells suggest that palmitoylation is in some way dependent
on cholesterol addition, as only a small fraction of a form
of Shh-N that lacks cholesterol, generated by a mutant form
of the cDNA, is palmitoylated (Pepinsky et al. 1998).
- In line with this, the unmodified form of Shh can elicit
equivalent responses in some in vitro assays when administered
at significantly higher concentrations (20–30•)
than mature native protein. In other contexts, however,
notably the ventralization of neural plate explants, both
acylated and unmodified forms of the protein appear to have
equivalent or very similar levels of activity (Pepinsky
et al. 1998; Kohtz et al. 2001). Replacement of the N-terminal
Cys by a hydrophobic residue is itself sufficient to increase
signaling activity, indicating that it is the hydrophobicity
per se, rather than the specific nature of the palmitoyl
moiety, that potentiates activity (Taylor et al. 2001).
In Drosophila embryos, Hh accumulates in characteristic
membrane-associated patches (Taylor et al. 1993; Tabata,
1994) that most likely
correspond to lipid rafts (Rietveld,
1999), that is, membrane microdomains that function
as platforms for intracellular sorting and signal transduction.
The lipid modifications of Hh may play a role in targeting
them to rafts; testing this proposition will require a comparison
of the subcellular localization and trafficking of the modified
and unmodified forms of the protein.[Ingham, 2001]
- Hh-Np can freely traverse cells lacking the Hh-binding activity of
Ptc, before being bound and endocytosed by ptc in genetically wild-type
cells (Chen, 1996).
- This sequestering activity of Ptc helps explain why the ptc gene
itself is a target of Hh activity: by up-regulating ptc transcription,
Hh effectively promotes its own sequestration, a negative feedback
mechanism that restrains the spread of Hh protein from its source
(Chen, 1996).
- Hh-Nu appears to be immune to ptc sequestration impling that
ptc sequestration depends critically on the cholesterol moiety in
Hh-Np (Chen, 1996).
- This might suggest that cholesterol mediates interaction between
Hh-Np and Ptc, perhaps via the latter’s SSD, several findings
argue against this:
- Not least of these are the facts that Hh-Nu
efficiently activates the pathway by abrogating Ptc activity
and that lipid modification has no significant effect on the
in vitro binding affinity of Hh for Ptc. Moreover, Hh-Nu appears
to be endocytosed with Ptc in responding cells (Burke,
1999), all of which raises questions about the basis of
Hh sequestration. Remarkably, whereas recent studies in chick
embryos suggest that Ptc1 has a similar role in sequestering
Shh in the vertebrate neural tube (Briscoe et al. 2001), in
vivo analysis of an unmodified form of Shh-N (Shh-Nu) reveals
a quite different effect of cholesterol modification on its
behavior (Lewis et al. 2001). In this case, absence of the cholesterol
moiety severely limits the range of the Shh-Nu protein. Therefore,
although it retains activity comparable to that of the wild-type
protein at short range (as assayed by its ability to promote
normal hair, whisker, tooth, and lung development and to promote
the specification of the most posterior digits in the hand and
foot plates), it fails to spread across the developing limb
bud, leading to a contraction in the expression domains of target
genes and an accompanying loss of intermediate digits (Lewis
et al. 2001). These findings point, instead, to a requirement
for cholesterol modification for the efficient movement of Shh-N
through the limb mesenchyme, a requirement that seems at odds
with the properties of the Hh-Nu form in Drosophila. It is possible
that this disparity may reflect a difference in the experimental
conditions under which the unmodified forms of the respective
proteins have been assayed, or in the cellular milieu in which
the endogenous forms normally operate (Lewis et al. 2001), but
it is also notable that in vertebrates, Hh proteins are subject
to an additional restraining influence, namely, that imposed
by Hip1 (Chuang and McMahon 1999), an Hh-binding protein that
has no counterpart in Drosophila. Like Ptc, expression of Hip1
is up-regulated in response to Hh signaling (Chuang and McMahon
1999), but unlike Ptc, there is no evidence that it acts by
directly regulating Smo. Therefore, Hip1 adds a second layer
of control to the Hh negative feedback mechanism, a layer that
is exclusive to vertebrates. One scenario that could reconcile
the immunity of Drosophila Hh-Nu to Ptc sequestration with the
attenuated range of Shh-Nu in the mouse would be if Shh-Nu were
to bind Hip with equal or greater affinity than its cholesterol-coupled
counterpart. In this case, Shh-Nu would still be capable of
antagonizing Ptc at short range, but would not be free to move
beyond cells immediately adjacent to its source. But how might
cholesterol coupling allow Shh-Np to override Hip sequestration?
In Drosophila, movement of the cholesterol-modified form of
Hh-Np depends critically on the activity of tout velou (ttv)
(see Fig. 7; Bellaiche et al. 1998), a homolog of the human
EXT genes that were identified through their association with
the bone disorder multiple exostoses (Stickens et al. 1996).
These genes encode GAG transferases (Lind et al. 1998), implying
that TTV (and its vertebrate homologs) generates a proteoglycan
that mediates the transfer of Hh-Np between cells (Bellaiche
et al.1998; Thé et al. 1999). That the activity of TTV
is required in Hh-receiving cells even in the absence of Ptc
(Bellaiche et al. 1998) implies that the hypothetical proteoglycan
may interact directly with Hh-Np and possibly present it to
Ptc, a process similar to that proposed above for Hip. If both
the proteoglycan and Hip compete for Hh-Np binding and if cholesterol
coupling is obligate for interaction with the former, then it
is easy to envisage how in vertebrates, the absence of cholesterol
from Shh-Nu would block its movement and lead to its sequestration
by Hip. In Drosophila, in contrast, where there is no Hip1 to
bind unsequestered Hh-Nu, the latter might remain free simply
to diffuse away from its source, abrogating Ptc activity in
its wake. Investigating the properties of Shh-Nu in a Hip mutant
background should therefore help to resolve this issue. [Ingham,
2001]
|
|
|
- Mouse homologue: Sonic hedgehog, Desert hedgehog, Indian hedgehog
(Echelard, 1993)
- Dhh is most closely related to Drosophila hedgehog. Ihh and Shh
are more related to one another, representing
a more recent gene duplication event.
- hh genes have been identified in several other invertebrate species
including the leech and sea urchin (Chang et al.1994) as well as in
the cephalochordate amphioxus (Shimeld 1999).One notable exception is
the nematode worm Caenorhabditis elegans ,which has no hh ortholog (Aspock
et al.1999)but does possess severa lgenes encoding proteins homologous
to the Hh receptor Patched (Kuwabara et al.2000) [passage taken from
Ingham and McMahon, 2001]
- Vertebrate Hh homologues play key roles in the morphogenesis of the
neural tube, somites, axial skeleton, limbs, lung and skin (reviewed
in Neumann and Cohen, 1997; Ingham, 1998; Ruiz i Altaba, 1999; McMahon,
2000)
- Structural analysis of Hh-N provided initial excitement as it revealed
a striking conservation with zinc hydrolases, suggesting that the Hh
ligand might have an enzymatic activity (Hall et al. 1995). However,
an absence of conservation of key histidines that coordinate the zinc
ion in hydrolases and biochemical analyses in cell culture seem to argue
against an enzymatic role for the signaling moiety (Fuse et al. 1999).
|
|
|
- hh mutant embryos are short and show a sever 'lawn of denticle' phenotype
(Nusslein-Volhard, 1984)
- If Hh signal is not transmitted, levels of ptc expression and apparent
levels of Ci protein are not elevated, dpp is not transcribed, and Fu
protein is not phosphorylated (Tabata,
1992; Basler,
1994; Capdevila,
1994; Felsenfeld and Kennison, 1995; Sanicola et al., 1995 Therond,
1996)
- Reduction of the hh dosage (hhts2) enhanced
the partial fusion of L3 and L4 observed in kn1/col1
wings (Vervoort,
1999)
- hhts2 is a temperature sensitive allele. It behaves
as a null at 30°C and as a strong hypomorphic mutant at 25°C
(Strigini,
1997; Ma,
1993)
- in a gain of function allele called Moonrat (hhMrt) hh
in addition to its normal expression in the posterior compartment of
the wing disc, is ectopically expressed along the DV boundary within
the anterior compartment (Felsenfeld and Kennison, 1995)
|
Overexpression
/ Ectopic expression |
- uba1>hh clones in the A compartment express dpp-lacZ but not hh-lacZ
and do not have smooth borders like Tuba1>en clones do (Zecca,
1995)
- Overexpression or misexpression of hh (UAS-hh/en-Gal4 or UAS-hh/apterous
(ap)-Gal4) led to an expanded expression of col anteriorly (Figure 4c),
or ectopic expression dorsally (Figure 4d), respectively. (Vervoort,
1999)
- In embryos overexpressing hh with a HS-hh construct
wg is ectopically expressed anterior to each normal wg domain,
but more anterior cells don't express wg. ptc, however,
is expressed in all cells except engrailed expressing cells.
(Ingham,
1993)
- The distribution of En protein is unaltered in heat-shocked HS-hh
embryos (Fig 2C). (Ingham,
1993)
- In embryos overexpressing hh with a HS-hh construct
the ventral denticles characteristic of the posterior part of each belt
are eliminated and replaced by those typical of the second and third
row of wild-type belts (Fig 3a, d) (Ingham,
1993). The denticle belts are also reduced in the lateral extension
so that they become less trapezoidal and more rectangular. This phenotype
is very reminiscent of that of ptc mutants.
|
|
|
| {Reagents} |
|
|
|
|
|