| |
| |
|
|
| |
|
|
| Symbol: Ci
|
Flybase ID: FBgn0004859 |
| Synonyms: ci dominant
|
GadFly |
| Function: {Short_Function} |
{LocusLink} |
| Keywords: {Keywords} |
{Interactive_Fly} |
A Zinc Finger transcription factor that is the transcription
factor of the Hedgehog signaling pathway.
|
|
|
- "Ci is absolutely required for all examined aspects of Hh outputs"
(Methot,
2001)
|
|
|
- Flybase
list of genetic interactions
- genes activated by Ci-155
- genes repressed by Ci-75
- Ci Proteolysis
- PKA catalytic subunit increased proteolytic processing (Chen,
1998)
- PKI, a PKA inhibitor, blocks processing (Chen,
1998)
- Blocking processing w/ proteasome inhibitors or altered Ci proteins,
is insufficient for activation of Hh targets (Chen,
1999)
- ci protein levels in fu mutants (fu94) remain inappropriately
high and uniform across the anterior compartment during stage 11
(Fig 4A,B) (Motzny,
1995).
- ci protein levels in fu mutants (fu94) are high and
uniform across the anterior compartment (Fig 7) (Slusarski,
1995)
- in hh mutants, the distribution of the ci protein across the segmental
stripe of expression also remains uniform beyond stage 11 but the
ci protein levels are not detectably different than wild type (Fig
5B) (Motzny,
1995).
- Enhancers
- A minimal dpp disc enhancer was able to respond to Ci-155 and
Ci-75 and that their activities can be replaced by a single synthetic
Gli-binding site (Muller,
2000)
- The enhacer sequences of ptc effectively integrates the repressor
activity of Ci if placed into a dpp context (Muller,
2000)
- Necessary for the dppho enhancer but not sufficient (Hepker,
1999)
- costal-2
- CREB
- Ci requires CREB-binding protein to activate wg expression in
the embryo (Chen,
2000)
- decapentaplegic
- both the domain and levels of transcription of dpp, which ultimately
determine the size and pattern of the adult appendage, are controlled
by the balance between the Hh-dependent activator and repressor
activities of Ci (Methot,
1999)
- fused
- hedgehog
- Ci is the mediator of Hh-dependent transcriptional activation
(Forbes,
1993)
- ci clones express low levels of hh irrespectively of their position
(Dominguez,
1996)
- hh mutant embryos have a stronger phenotype than ci mutants because
hh mutants produce Ci-75 repressor (Methot,
2001)
- patched
- clones doubly mutant for ptc and ci exhibit the same wing defects
(see mutations) as ci single mutant clones. (Methot,
2001)
- ptc ci embryos show a higher degree of naked cuticle compared
with ci mutants (Fig 6K) indicating some Hh-transducing activity.
This is due to maternally provided Ci. This maternal Ci protein
is apparently inconsequential when Hh is secreted normally by en-expressing
cells, but it may be relevant when the pathway is prematurely activated
in ptc mutant animals (Methot,
2001)
- embryos that lack maternal ci in addition to zygotic ci and ptc
are indistinguishable from embryos lacking only ci (Fig 6H-J) (Methot,
2001)
- PKA
- transcriptional activity of Ci
- PKA inhibits an uncleavable form of Ci, CiU --> shows that
PKA negatively regulates the transcriptional activity of the full-length
Ci independent of Ci cleavage (Wang,
2000)
- Mutating PKA phosphorylation sites in CiU makes it more active
(Wang,
1999)
- Ci is more active in PKA clones than in slimb clones (Wang,
1999)
- Exogenous PKA can increase further the transcriptional activity
of the ci PKA mut (Chen,
1998)
- wingless
- Ci-75 is needed to repress wg latter in embryo development
- ci single mutant and ci hh double mutant embryos are close
to wild-type size and show considerable naked cuticle ventrally,
ci wg double mutants are small and exhibit a 'lawn-of-denticle'
phenotype (Methot,
2001)
|
|
|
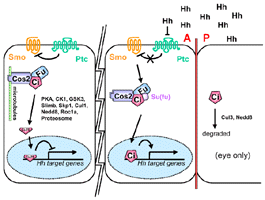
- Ci signaling complex
- Cos-2-Fu-Ci complex associates w/ microtubules in absence of Hh
signalling (Robbins,
1997, Sisson,
1997)
- Cos2 and Su(fu) inhibit Ci by tethering it in the cytoplasm, wheras
Hh induces nuuclear translocation of Ci through Fu (Wang,
2000)
- Complex probably contains Su(fu) (Monnier,
1998)
- Ci-Cos2
- Cos2 binds Ci’s 941-1065aa and prevents its nuclear import,
and inhibites its activity via this domain (Wang,
2000)
- Ci-155 and Ci-75 both coelute w/ Cos-2 and Fu (Aza-Blanc,
1997)
- Ci-Su(fu)
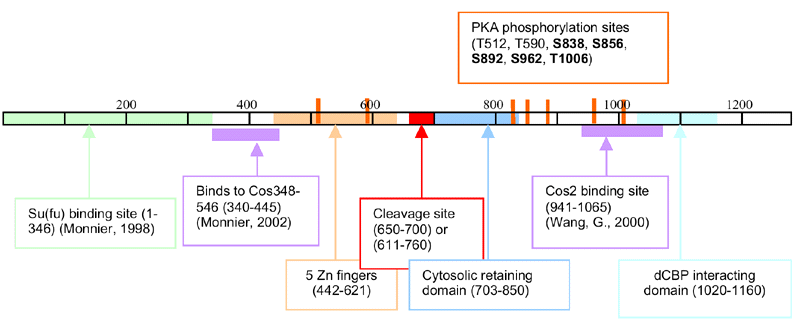
- Ci’s aa 1-346 was sufficient for binding to Su(fu) (Y2hybrid
and GST) (Monnier,
1998)
- Ci-Fu
- Residues between 703 & 850 tether Ci in the cytoplasm (Aza-Blanc,
1997)
- Ci-DNA
- Binding sites have been identified upstream of the promoter both
of wg and ptc
- Binds the following sequence:
TGGG(T/A)GGTC
C-----C------
G-----------A
-A---------C-
-T-----------
-----------CG
|
Transcriptional
Regulation |
- is independent of Hh
- transcribed uniformly in all cells that are responsive to Hh
- En/Inv negatively regulates ci expression (Eaton,
1990)
|
|
|
- C-term has an acidic (transcription activation domain) and CBP binding
site
- Amino Acids - 1282
- In the middle of Ci are 5 tandem C2H2 Zn fingers (Orenic,
1990)
- an NLS
- # of consensus PKA phosphorylation sites that have been conserved
in Gli proteins
- S: mutating phosphorylation sites inc. stability in Ci
- Zn finger domain is sufficient for its target specificity (Alexandre,
1996)
- "Cleavage site" 650-700 (Aza-Blanc,
1997) or 611-760 (Methot,
1999)
|
Location (protein
and transcript) |
- Full length is cytoplasmic (Motzny,
1995)
- Ci76 is nuclear (Aza-Blanc,
1997)
- N-term has nuclear localization and DNA binding characteristics, residues
between 703 and 850 tether Ci in the cytoplasm (Aza-Blanc,
1997)
- Hh signaling increases the rate of Ci155 nuclear import, resulting
in significant nuclear accumulation (Chen,
1999)
- Even in the absence of signaling, nuclear accumulation of Ci155 (LMB
treatment) suffices for significant induction of Hh targets (Chen,
1999)
- A previously identified cytoplasmic localization domain of Ci155 (residues
675-860) (Aza-Blanc,
1997) actually constitutes a nuclear export signal (Chen,
1999)
- Ci protein localization in drosophila embryo visualized by 2A1 antibody
(recognizes full length Ci)
- Ci protein first appears dorsally in the cellular blastoderm of
embryos (Fig 1A). (Motzny,
1995).
- The expression domain expands throughout the cellularized embryo
and remains uniform up to germ band extension (stage 8). At this
time, ci protein becomes restricted into segmentally repeated stripes
[due to repression by en in the posterior compartment (Eaton,
1990)]. (Motzny,
1995).
- Through early stage 10 the pattern of ci protein expression closely
follows that of the transcript (Fig 1B) (Motzny,
1995).
- By stage 11 ci protein expression changes relative to that of
the transcript. The middle portion of each stripe of ci protein
shows decrease antibody labeling relative to the borders (Fig 1C)(Motzny,
1995).
- In imaginal discs there are elevated levels of ci protein just anterior
to the compartment boundary, with lower uniform levels throughout the
remainder of the anterior compartment (Fig 2A-C) (Motzny,
1995).
- Ci antibody staining using either monoclonal or polyclonal antibodies
is localized primarily in the cytoplasm. Low levels of ci protein were
detected when compared to the nuclei of en expressing cells (Fig 3)
(Motzny,
1995).
|
Protein
Modifications and Regulation |
- Proteolysis
- Requires:
- Cos-2
- cells laking Cos-2 accumulate Ci-155 independently of
Hh activity and inappropriately activate Hh target genes
(Wang,
2000)
- Formation of Ci-75 requires Cos2, PKA and C-term of Fu
(Methot,
2000)
- Hh
- when cells receive Hh, cleavage of Ci is blocked and the
cytoplasmic tethering domain is retained (Aza-Blanc,
1997)
- Ci75 is present in all Hh-responsive cells, Ci-155 accumulates
specifically in cells in close proximity to those secreting
Hh (Motzny,
1995; Aza-Blanc,
1997; Wang,
1999), strongly suggesting that Hh acts by inhibiting
its proteolytic cleavage.
- Ci-ubiquitin conjugates could be detected in tissue culture
with overexpression of differently tagged Ci and ubiquitin
proteins in cl-8 cells, the formation of such conjugates
was not under Hh regulation and could not be detected when
Ci protein was expressed at physiological levels (Chen,
1999)
- Hh signaling in general
- Hh signaling reduces phosphorylation of Ci155, which appears
to decrease processing in cultured cell line (Chen,
1999)
- Fused
- Formation of Ci-75 requires Cos2, PKA and C-term of Fu
(Methot,
2000)
- PKA
- cells laking PKA accumulate Ci-155 independently of Hh
activity and inappropriately activate Hh target genes (Ohlmeyer,
1998).
- levels of unproteolysised
protein that accumulate in the absence of PKA activity
overwhelm the sequestration mechanism, so that some
uncleaved protein is able to enter the nucleus and activate
Hh target genes (Wang,
2000).
- however, the response of cells
to loss of PKA (as assayed by the activation of Hh target
genes) is stronger than that when Cos-2 activity is
removed (Ohlmeyer,
1998; Wang,
2000). This implies that phosphorylation has an
additional effect on Ci, not only priming the protein
for cleavage but also attenuating its activity, an effect
that could be abrogated by the activity of an Hh-regulated
phosphatase. Presumably in the absence of Cos-2, the
full-length Ci remains phosphorylated on these critical
residues (although this has not been established) and
hence is not fully active. [Ingham, 2001]
- mutation of one or more of the PKA sites in Ci is sufficient
to inhibit its proteolysis (Chen et al. 1998).
- Ci can directly be phosphorylated by PKA in vitro (Price,
1999)
- mutant form of Ci lacking 5 potential PKA phosphorylation
sites (Ci5m) does not have detectable cleavage into Ci-75
(Price,
1999)
- changes in PKA activity alters Ci-155 levels and not Ci5m
(Price,
1999)
- Ci5m remains Hedgehog responsive if expressed at low levels
(Price,
1999)
- Formation of Ci-75 requires Cos2, PKA and C-term of Fu
(Methot,
2000)
- Proteasome
- depends on proteasome activity (proteasome inh, &
deleting cleavage site), but blocking proteolysis alone
cannot account for the full induction of Hh target genes
(Chen,
1999)
- cleavage requires Slimb (Jiang,
1998)
- Detected Ci-ubiquitin conjugates using cotransfection
and overexpression of differently tagged Ci and ubiquitin
proteins in cl-8 cells, however, the formation of such conjugates
was not under Hh regulation and could not be detected when
Ci protein was expressed at physiological levels (data not
shown) (Chen,
1999).
- Mechanism:
- Ci-75 is generated by a proteolytic cleavage event that removes
the transcriptional activation domain of the protein, yielding
a truncated protein that retains the zinc finger-binding domain
and an N-terminal repression domain (Aza-Blanc,
1997)
- Ci-155 Half-life=75 min in tissue culture (Aza-Blanc,
1997)
- Ci76 (and presumably Ci75) acts as a repressor and represses
Hh expression (Aza-Blanc,
1997)
- Independent of nuclear localization
- Blocking processing did not lead to nuclear localization
(Chen,
1999)
- Cleavage is necessary for repressor function (Methot,
1999)
- Cleavage is essential for limb patterning and is regulated by
Hh in vivo (Methot,
1999)
- repressor activity of Ci is subject to Hh regulation (Methot,
1999)
- activity of an unproteolysisable form of Ci requires additional
Hh-dependent regulation
- a form of Ci lacking the protease-recognition site, although
resistant to cleavage, is totally inactive except in cells stimulated
by Hh signaling (Methot,
1999; Wang,
2000).
- cells lacking the activity of the slmb gene (which encodes
an F-box protein implicated in priming substrates for proteasome-dependent
cleavage) accumulate full-length Ci, but fail to activate ptc
transcription (Wang,
2000).
- Nuclear Import
- Active nuclear export of Ci-155 is an essential mechanism for
maintenance of the unstimulated state (Chen,
1999)
- Hh signaling and target gene activation are associated w/ an inc
in the nuclear concentration of Ci-155 (Chen,
1999)
- Hh signaling increases the rate of Ci-155 nuclear import (Chen,
1999)
- Even in the absence of signaling, nuclear accumulation of Ci-155
(by LMB) suffices for a slow but significant induction of Hh targets,
and active nuclear export of Ci-155 is an essential mechanism for
maintenance of the unstimulated state (Chen,
1999)
- LMB failed to induce higher levels of reporter expression when
combined with Hh, even though the nuclear levels of Ci155 resulting
from such a combined treatment are somewhat higher (Chen,
1999)
- This might suggest that there is a constitutive change in Ci to
export it back out of the nucleus and that this form is not as active
- perhaps dCBP plays a role in nuclear
transport of Ci (Akimaru,
1997)
- "Activation"
- Evidence for the existence of a distinct activator form of Ci,
which does not arise by mere prevention of Ci proteolysis, but rather
depends on a separate regulatory step subject to Hh control (Methot,
1999)
- Hh signaling is necessary for the full-length form of Ci to function
as activator (Methot,
1999)
- In the wing, ptc expression, as well as a late phase of Hh-dependent
en expression, is controlled solely by the Ci activator (Methot,
1999)
- Proteolysis
- Ci-ubiquitin conjugates could be detected in tissue culture with
overexpression of differently tagged Ci and ubiquitin proteins in
cl-8 cells, the formation of such conjugates was not under Hh regulation
and could not be detected when Ci protein was expressed at physiological
levels (Chen,
1999)
- In absence of Hh, Ci is cleaved after Zn finger domain to generate
a 75kD form (Ci-75)
- Phosphorylation:
- Phosphorylation at the PKA sites primes Ci for phosphorylation
by GSK3 (Jia,
2002, Price,
2002) and CK1 (Price,
2002). This then leads to Ci proteolysis (Jia,
2002; Price,
2002).
- Several studies identified phosphorylation of Ci by protein kinase
A (PKA) as a key event in mediating cleavage (Chen,
1998; Chen,
1999; Price,
1999; Wang,
1999; Wang,
1999).
- Ci protein resistant to phosphorylation by PKA is still subject
to Hh control (Methot,
2001) b/c it’s still tethered in the cytoplasm.
- phosphorylation of Ci promotes its proteolysis (Chen,
1998)
- Okadaic acid, a phosphatase inhibitor shifted the balance in favor
of slower-migrating Ci species (Chen,
1999)
|
|
|
- Seq: human/mammalian GLI proteins: Gli1, Gli2, and Gli3 (after glioblastoma)
- C. elegans tra-1
|
|
|
- Ci PKA site mutants
- a mutant form of Ci lacking five potential PKA phosphorylation
sites (Ci5m) is not detectably cleaved to Ci-75 in Drosophila embryos
(Price,
1999)
- When all Ci’s PKA sites are mutated Ci is not processed and
has increased transcriptional activity (Chen,
1998)
- ci mutant wing clones have wing perturbations
- ci mutant clones cause wing defects:
- Vein L2 perturbations
- Vein L3 duplication and forking
- Intervein L3-L4 margin contains sockedted bristles which are normally
found in the anterior compartment.
- Vein L4 has defects
- Vein L4 often bear ectopic campaniform sensilla
-
- ci-/- embryos derived from ci94 mutant germline clones (Fig 6I) are
indistinguishable from ci-/- embryos derived from heterozygous mothers
(Fig 6H) (Methot,
2001)
- Ci clones fail to upregulate ptc and en expression but do express
low levels of dpp and hh irrespectively of their position (dpp &
hh: Dominguez,
1996) (Methot,
1999)
- Mutation in the PKA (###), GSK3 (Jia,
2002; Price,
2002), and CK1 (Price,
2002) sites leads to inhibition of Ci proteolysis.
- CiC = amino acids 612–712 were removed and is still proteolysis
to Ci-75 (Methot,
1999)
- CiU = amino acids 611–760 were removed and is no longer proteolysis
to Ci-75 (Methot,
1999)
- can substitute for embryonic Ci in spite of the fact that it cannot
form detectable amounts of Ci-75 rrepressor
- Anterior clones doubly mutant for ci94 and enE
behave in all respects like ci94 single mutant clones (Méthot
and Basler, 1999) and express low levels of dpp (Fig. 4A). Clones lacking
ptcIIW and enE exhibit very high levels of dpp
expression, regardless of their position within the wing imaginal disc
(Fig. 4B). In ptc en ci triple mutant clones, the levels of dpp expression
reverted to those found in ci en double mutant or ci single mutant anterior
clones (Fig. 4C). (Methot,
2001)
- They noted one difference between ci en and ci ptc en clones.
Non-autonomous dpp expression is detected only in a few wild-type
cells surrounding ci en clones. In contrast, widespread non-autonomous
dpp transcription is often associated with ci ptc, or ci ptc en
clones (Fig. 4 and data not shown). This difference is due to the
reduced ptc+ dosage as these clones were generated in ptcIIW/+
heterozygous animals. A twofold difference in levels can cause a
discernible effect on the ability of Ptc to sequester Hh or inhibit
the signaling activity of Smo (Chen et al., 1996; data not shown).
No differences, however, was observed within ptc en ci versus en
ci mutant clones. From this they conclude that maximal activation
of the Hh pathway by removal of ptc has no effect on dpp transcription
if Ci is lacking. (Methot,
2001)
- alleles:
- ci94 null mutation contains a 5 kb deletion within
the ci locus that removes the promoter and the first exon of ci
including the sites for transcriptional and translational initiation
(Methot,
1999). No Ci protein can be detected immunologically (Slusarski
et al, 1995; Methot,
1999)
- Homozygous ci94 mutant embryos die and display
a segment polarity defect with a deficit of naked cuticle (although
not as severe as hh mutants) (Slusarski,
1995)
- CiCell is a mutation that acts like the repressor form of Ci,
is truncated at aa 975 (Methot,
1999)
|
Overexpression
/ Ectopic expression |
- Elevated levels of Ci are sufficient to activate ptc and other hh
target genes, even in the absence of hh activity (Alexandre,
1996, Dominguez,
1996; Hepker,
1997)
- Maybe high levels of Ci titrated out negative
regulators of Ci
- high levels of ectopic Ci-155 in the posterior compartment (achieved
by using either UAS-ci/en-Gal4 or the dominant ciW and ci1 mutations)
resulted in ectopic col expression (Figure 4e). (Vervoort,
1999)
- UAS-driven expression of Ci-76, in a domain overlapping that of col
expression (ptc-Gal4 or dpp-Gal4 drivers) prevented col expression (Figure
4f). (Vervoort,
1999)
- UAS-CiU [a stabilized form of Ci] driven by ptc-GAL4 resulted in activation
of Col in cells corresponding to the prospective wing margin (compare
Figs. 6F and 6G). Also the adult wings of such exhibit variable defects
at their distal tips, from notches (Fig. 6K and arrowhead in Fig. 6L)
to absence of bristles with remaining vein tissue (Figs. 6L and 6M)
(Glise,
2002)
- This latter phenotype is reminiscent of the effects of weak hypomorphic
wingless alleles that affect bristle formation without notching
(Couso et al., 1994).
|
|
|
- Ab to N-term: 1C2
- Ab to C-term 2A1
|
|
|
|
|
|