| |
| |
|
|
| |
|
|
| Symbol:
{Links}
|
Flybase ID: {Flybase_ID} |
| Synonyms: {Name}
|
{GadFly} |
| Function: {Short_Function} |
{LocusLink} |
| Keywords: {Keywords} |
{Interactive_Fly} |
{Summary}
|
|
|
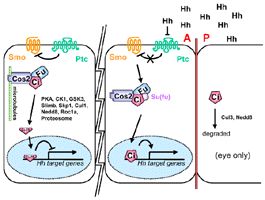
- Is a serine/threonine kinase
- At A/P boundary Fu kinase activity is involved in
maintenance of high ptc and late en (Alves,
1998), b/c it is needed for Ci to go into the
nucleus (Wang,
2000)
- In more anterior cells Fu plays a role independent
of its kinase fxn in regulating Ci stabilization (Alves,
1998) and nuclear import (us)
- Fu fxn is necessary for the nuclear import of Ci
and counteracts the negative Su(fu) effect on the
pathway.
- Phosphorylates Cos2 on S572 and to a lesser extent
S931 in a Hh dependent fashion (baculovirus system)
(Nybakken,
2002)
- Fu is able to autophosphorylate itself in baculovirus
system (Nybakken,
2002)
- Fu is acting downstream of ptc and hh, and upstream
of cos2 and ci and possibly pka in imaginal discs
(Sanchez-Herrero,
1996)
|
|
|
- Hedgehog
- transcription of hh persists for longer
in embryos lacking wild-type fu activity (Fig
2a), suggesting that fu may act 'downstream' of
hh to regulate wg transcription (Ingham,
1993)
- Wingless
- in a fu mutant there is no expression
of wg in the ventral ectodermal cells of
each parasegment in the embryo, though transcript
is still found in other cells where wg
expression is independent of hh function.
The phenotype stays the same even if Hh is overexpressed
with HS-hh. (Ingham,
1993)
- Ptc and Fu in the germarium undergo changes in expression
that are coincident with Sxl (Vied,
2001)
- Shown to act upstream of Ci (Slusarski,
1998)
- Fu mediates hh-induced en activation in the anterior
compartment in wing discs (Sanchez-Herrero,
1996) b/c Fu is need for release (us)
- Class 0, I, and II fused phenotype is suppressed
by Su(fu)LP (class II has a cos2 phenotype in addition)
(Preat,
1993)
- cos23 mutation partially suppresses fu vein phenotype
just like Su(fu)LP (Preat,
1993)
- fu[I]; cos27 flies look like cos27 flies
(Preat,
1993)
- fu[II]; cos23 die as late pupae and have
extreme adult cos2 phenotype (Preat,
1993)
- class II is recessive to fu+; class II
is recessive to class I; class 0 is recessive to class
II
- In the embryo Fu product can correct the deficiency
in the Hh pathway observed in fu mutants when expressed
only in the anterior neighbouring cells of Hh, which
are the wg-expressing cells (Thérond,
1999)
- Forced expression of Fu in S2 cells stimulates Hh-triggered
and Ci-dependent transcriptional activation (Fukumoto,
2001)
- N-term kinase domain is required for this activity
but the C-term is not (Fukumoto,
2001)
- Threonine 158 is essential for Fu activity; may
be involved in the activation of the kinase catalytic
activity (Fukumoto,
2001)
- fu phenotype is exacerbated by reducing col
dosage (Vervoort,
1999)
- In fu1 mutant discs, col expression at the A/P boundary
was interrupted at the dorso-ventral margin (Figure
5c), correlating with local fusion of veins L3–L4
at the margin (Figure 5a). (Vervoort,
1999) kn medial expression in the wing
pouch is reduced in fu mutants (Mohler,
2000)
|
|
|
- Immunoprecipitations from ovarian extracts show
that Sxl is in a complex with Fu and Cos2, along with
b- and g-tubulin (Vied,
2001)
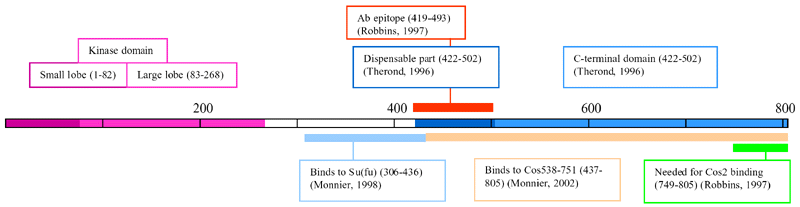
- The C-term domain (aa 421-805) of Fu is necessary
and sufficient to bind Cos2 (Ascano,
2002)
- The C-term domain can compete with endogenous Fu
for binding to Cos2 (Ascano,
2002)
- There appears to be a separate Cos2-Fu species in
gel filtration experiments (Ascano,
2002)
- aa 306-436 was sufficient for binding to Su(fu)
(Monnier,
1998)
- Ci and Fu co-IP (Robbins,
1997)
- Ci interacts w/ Fu-reg through Su(fu) (Monnier,
1998)
- Cos-2-Fu-Ci complex associates w/ microtubules in
absence of Hh signaling (Robbins,
1997, Sisson,
1997)
- complex is made irrespective of Fu and Cos-2phosphorylation
(Robbins,
1997)
- Complex probably contains Su(fu) (Monnier,
1998)
- Fu-P might preferentially associate w/ Cos-2 (Robbins,
1997)
- Functional Fu kinase is probably not necessary for
Fu and Cos-2association (Robbins,
1997)
|
Transcriptional
Regulation |
- Fu protein is throughout wing disc, but higher in
A
|
|
|
- N-term (1 - ~268aa) domain has ~30% identity with
catalytic domain of serine-threonine kinases (Therond,
1993)
- C-term domain has no significant similarity with
any known protein and may represent the regulatory
domain of the kinase (Therond,
1993)
|
Location (protein
and transcript) |
- Fu transcripts are uniformly distributed in embryo
(Thérond,
1993)
- Fu protein is present throughout the entire wing
disc, but its level is much higher in the anterior
compartment (Fig. 1D Alves,
1998)
- Fu and Cos2 can enter the nucleus, but the rate
of entry does not change with Hh signaling (Methot,
2000)
|
Protein
Modifications and Regulation |
- Becomes phosphorylated in both embryos and S2 cells
in response to Hh activity (Therond,
1996)
- becomes hyperphosphorylated (on serines) in response
to Hh activity
- appears as long as 30 minutes after induction,
suggesting that it represents a feedback device
rather than an event in initial signal transduction.
This leads in turn to the possibliity that Fused
is not autophosphorylating, even though the phosphoryation
can be abolished by mutations in the catalytic
domain of Fused. Similarly, Fused is apparently
not directly responsible for the phosphorylation
of Cos2, which occurs even when inactivating mutations
are present in the kinase domain of Fused (Robbins,
1997).
|
|
|
|
|
|
|
- Class 0: large deletions encompassing the
whole fu gene and some neighboring genes (Preat,
1993)
- completely suppressed for all their mutant phenotypes
by Su(fu)LP (Preat,
1993)
- Class I: mutation in kinase domain that doesn’t
affect open reading (Preat,
1993)
- completely suppressed for all their mutant phenotypes
by Su(fu)LP (Preat,
1993)
- Class II: mutates regulatory domain (Preat,
1993)
- although suppressed for their fused phenotype,
display a new maternal and zygotic phenotype with
Su(fu)LP, which looks like cos-2
mutations (Preat,
1993)
- Class dominace I > II > 0 : class mutants
are dominant over class II in fuII; Su(fu)LP
flies whereas class 0 mutants are recessive over class
II in fu0 / fuII; Su(fu)LP
- Fu phenotype:
- embryos:
- fuII; Su(fu)LP embryos
lack the median anterior part of each segment
with mirror-image duplication of the remaining
most anterior structures (Preat,
1993)
- adult wings:
- reduction of the 3-4 intervein region; anterior
double row bristles reach the fourth vein
- Only fu- clones located between veins 3
and 4 generate a mutant phenotype; fu clones
never effected vein 4 (Alves,
1998)
- fu- clones never crossed the A/P boundary
(Alves,
1998)
- fuII; Su(fu)LP adults
display duplication of the wing and leg anterior
compartments (Preat,
1993)
- A lot of fu mutations are in (Therond,
1996) see also: FlyBase
Report
- Mutations in the extracatalytic domain abolish both
the biological fxn of Fu and its association w/ Cos-2
(Robbins,
1997)
- Eliminating Fu kinase activity reduces Hh-target
gene expression while inc. [Ci-155]
- The absence of late en expression in fu mutants
could account for the expansion of the dpp stripe
up to the A/P boundary and for the concomitant extension
of the double row bristles (Alves,
1998)
- In fu mutants Ci accumulates at a high level in
a 10 cell wide stripe which displays a sharp posterior
limit along the A/P border (Alves,
1998) fuA and fu94 have high levels throughout
the A (Alves,
1998, Wang,
2000)
- Fu mutations suppress the effects of ectopic hh
expression (Alves,
1998)
- In fu mutants late en expression is lost which might
result in the wider dpp stripe due to absence of en
(Sanchez-Herrero,
1996)
- In fused mutant wing discs Col and Ptc expression
(protein and transcript) is absence approximately
six rows of cells on either side of the DV boundary
(Fig 1) (Glise,
2002) and the stripe is broadened due to increased
range of Hh in fused mutants (Fig 1) (Glise,
2002)
- In fused mutant wing discs dpp expression
is down-regulated at the DV boundary, but this is
not detectable by dpp-lacZ staining (Fig 1) (Glise,
2002)
|
Overexpression
/ Ectopic expression |
- Overexpression using several strains that strongly
express Gal4 throughout the wing disc, did not lead
to any abnormalities in an otherwise wild-type background
(Alves,
1998)
|
|
|
|
|
|
|
|
|
|