| |
| |
|
|
| |
|
|
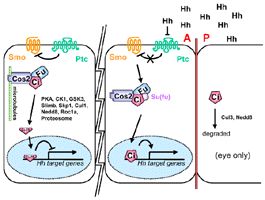
{Summary}
|
|
|
| {Function/Pathway}
|
|
|
- Smo acts downstream of hh and ptc (Alcedo,
1996)
- Hh mut & PKA mut had ptc RNA; Smo mut & PKA mut had no ptc
RNA -> suggest that smo has a significant basal activity in the absence
of Hh (Ohlmeyer,
1997)
- Little evidence implicating heterotrimeric G protein:
- In a classic assay for Galphai activation, expression
of human Smo in Xenopus melanophores appears sufficient to stimulate
persistent pigment aggregation in these cells, an effect that can
be blocked by pertussis toxin (DeCamp et al. 2000).
- In a primary fish myoblast assay system, the effects
of Shh were found to be insensitive to pertussis toxin (Norris et
al. 2000), and the results of treating zebrafish embryos with the
toxin are inconclusive (Hammerschmidt and McMahon 1998).
- Notably, there is to date no report of a G-protein mutation that
disrupts Hh signaling in Drosophila, despite several such mutations
having been isolated (e.g., Wolfgang et al. 2001).
- The M2 mutation of human Smo, isolated from a spontaneously arising
basal cell carcinoma, creates a constitutively active form of Smo.
This results in an amino acid substitution in the seventh transmembrane
domain predicted to disrupt G-protein coupling (Xie.
1998).
- A loss-of-function mutation in Drosophila Smo removes a charged
residue in the third intracellular loop; similar mutations in GPCRs
have been found to abolish G-protein coupling (S. Nystedt, H. Strutt,
and P.W. Ingham, in prep.).
- Genetic screens in mice identified a mutation, open-brain (opb), that
shows a phenotype opposite to that caused by loss of Shh activity (Gunther
et al. 1994; Eggenschwiler and Anderson 2000). Significantly, floor
plate and motor neurons, cell types that are absent in Shh mutants (Chiang
et al. 1996), differentiate in embryos mutant for both opb and Shh,
suggesting that the opb gene product acts downstream of Shh as a negative
regulator of the pathway. Nevertheless, expression of some Shh target
genes remains sensitive to Shh activity even in the absence of opb function,
indicating that loss of opb does not result in complete derepression
of the pathway (Eggenschwiler et al. 2001). Cloning of opb has revealed
that it encodes RAB23, a member of a large family of small GTP-activated
proteins associated with many dynamic aspects of membrane trafficking
(Eggenschwiler et al. 2001). Ptc1 could
negatively regulate Smo activity through a RAB23-dependent trafficking
process.
- smo is required for the response of cells to Hh signaling for embryo
and imaginal discs (van
den Heuvel, 1996)
- activation of Smo might modulate PKA activity (Alcedo,
1996), there is good evidence that Hh signaling has no effect on
PKA activity (Jiang,
1995). Therefore,it seems that phosphorylation by PKA is permissive
for Ci cleavage, the rate-limiting step being recruitment of Ci to the
microtubules. However, Hh might also contribute to cleavage regulation
by promoting the dephosphorylation
of Ci.
|
|
|
- Coimmunoprecipitates w/ ptc—unaffected by H
- Co-IP studies of vertebrate family members suggest that
Ptc and Smo interact directly to form a receptor complex that remains
associated after ligand binding (Stone,
1996; Carpenter, 1998; Murone, 1999)
- Evidence of IP Ptc/Smo complexes in Drosophila tissues
is lacking (Johnson, 2000)
- One model, based largely on analysis of the properties of the proteins
when overexpressed in tissue culture cells (Stone,
1996; Murone et al. 1999), suggests that Smo and Ptc interact directly
to form a membrane-associated receptor complex. The Smo present in this
complex is postulated to be inactive in unstimulated cells, but, upon
Hh binding to Ptc, the complex undergoes some conformational change
that results in the activation of Smo.
- It is notable, however, that in Drosophila, visualization
of the two proteins suggests that most Smo does not colocalize with
Ptc, at least in cells responding to Hh (Denef,
2000). Moreover, biochemical investigation of the postulated physical
interaction between the two proteins in vivo has so far proved negative
(Johnson et al. 2000).
|
Transcriptional
Regulation |
- Smo is dephosphyorylated by a type 2A protein phosphatase (Denef,
2000)
- Smo protein levels are upregulated posttranscriptionally by a Hh signalling
dependent mechanism. Inhibition of PKA, leads to upregulation of Smo
above just Hh signaling (Alcedo.
2000)
- Hh signaling upregulates Smo levels, which are otherwise down regulated
by Ptc (Alcedo.
2000)
- "Thus, we propose that Hh and its Ptc-Smo receptor have developed
the properties of a self-correcting system in which the Hh signal adjusts
the concentration of its receptor to its own concentration" (Alcedo.
2000)
- "Hence, it is crucial that Smo signaling strictly depends on
the presence of Hh and that, in the absence of Hh, constitutive Smo
signaling is restricted by Ptc below a threshold necessary for the transcriptional
control of Hh target genes" (Alcedo.
2000)
- "When Hh levels decrease, Smo is destabi- lized because of the
inhibition of Smo signaling by Ptc. The concentration of Smo will be
reduced more rapidly than that of Ptc, which continues to be translated
from a decreasing concentration of its mRNA, and eventually Smo will
reach a reduced steady-state concentration, which is lowest in regions
where Hh is absent. When the Ptc concentration falls below a threshold,
Smo signaling begins to inhibit its own degradation and to activate
transcription of ptc, whose product suppresses Smo signaling and thus
again downregulates itself and Smo. Hence, a new steady state is reached
at which the levels of Ptc and Smo are reduced to a level corresponding
to the low Hh concentration. The sequence of events are expected to
be reversed, if the Hh concentration is again increased. Thus, the Hh
signaling pathway has the properties of a self-correcting system, since
an imbalance between Ptc and Smo or between Hh and the Ptc-Smo receptor
is readjusted to equilibrium." (Alcedo.
2000)
- Since Smo signals constitutively in the absence of Ptc (Hooper, 1994;
Alcedo,
1996), Smo signaling must activate ptc to inhibit its constitutive
activity.
- "To avoid an imbalance between the two Hh- receptor moieties,
Smo signaling must also upregulate Smo. If Smo levels were independent
of Smo signaling, Smo would reach a uniformly high level while the concentration
of Ptc would oscillate around an equilibrium since Ptc inhibits Smo
signaling on which its synthesis depends. However, in this case Smo
would signal even in the absence or at low levels of Hh, which is not
what we observe (Figures 2C and 2D). Therefore, to ensure that Ptc and
Smo reach an equilibrium at which Ptc completely inhibits Smo signaling
most rapidly in the absence of Hh, Smo regulates its own breakdown."
(Alcedo.
2000)
- Genetic and molecular characterization of the smo gene (Alcedo,
1996)
- Has structural features of G protein-coupled receptors and is homolgous
to the frizzled gene—especially N term (Alcedo,
1996)
- C-term contains 5 potential PKA sites, 2nd intercellular loop has
a PKA site (Alcedo,
1996)
- Smo has constitutive signalling activity (Alcedo,
1996)
- Incubation of cells with concanamycin A, a specific vacuolar H+/ATPase
inhibitor that blocks transport out of early endosomal compartments,
protected Ptc1 from degradation
|
|
|
- Cloned Smo (van
den Heuvel, 1996)
- Sequence analysis: seven putative transmembrane domain, typical of
G-protein-coupled receptors, suggesting
that Smo may act as a receptor for Hh (van
den Heuvel, 1996)
|
Location (protein
and transcript) |
- Smo may be limited to a more basolateral domain (Denef,
2000)
- Expression location in embryos see (Alcedo.
2000)
- Smo protein accumulates specifically in cells in which Ptc activity
is absent or abrogated by Hh signaling, a process that seems to involve
the redistribution of a hyperphosphorylated form of the protein to the
cell surface (Denef,
2000) and may also be accompanied by a conformational change (Ingham,
2000)
- In KNRK cells in the absence of Ptc1, Smo accumulates at the cell
surface and in early endosomal compartments (Incardona,
2002)
- Internalization of Smo N-terminal antibodies by live cells resulted
in the labeling of structures indistinguishable from those labeled by
C-terminal anti-Flag in fixed, permeabilized cells (Figure 2B), suggesting
that intracellular Smo is derived by endocytosis (Incardona,
2002)
- juxtanuclear Smo showed no colocalization with the TGN marker TGN38
(Figure 3G) and instead colocalized with the transferrin receptor (Figure
2E). Finally, Smo did not colocalize with LBPA+ or LAMP-1+
late endosomes/lysosomes (Figures 2F and 2G), even in the presence of
leupeptin (Figure 2H). (Incardona,
2002)
- Treatment of cells with chloroquine
showed differences between the distributions of Ptc1 and Smo
- Ptc1 appeared in endosomes marked with fluid-phase tracer within
30–60 min of chloroquine addition (Figures 3B and 3C), prior
to the appearance of TGN38 in endosomes. By 90 min, Ptc1 and TGN38
colocalized extensively in endosomes (Figure 3D). The size and number
of Ptc1+/TGN38+ vesicles gradually increased
and were maximal between 2 and 3 hr of treatment (Figures 3E and
3F). In contrast, while TGN38 accumulated in endosomes, chloroquine
had little effect on the distribution of Smo (Figures 3G–3L).
However, after 6 hr in chloroquine, large, ring-shaped Smo+
vesicular structures were observed (Figure 4). Therefore, Smo either
undergoes much slower internalization than Ptc1 or is sorted from
the endocytic pathway at a relatively chloroquine-insensitive step.
ShhN had no effect on Smo distribution (data not shown). Shh had
no influence on the kinetics with which Ptc1+ vesicles
appeared in chloroquinetreated cells, and the colocalization of
Ptc and Shh in these vesicles was retained (data not shown). These
results confirm that Ptc1 reaches endosomes via the cell surface
rather than by a direct Golgi-endosome route, consistent with the
effects of concanamycin A. (Incardona,
2002)
|
Protein
Modifications and Regulation |
| {Modifications} |
|
|
- Resemblance to G protein-coupled receptor and members of the Frizzled
family of serpetine proteins
- belongs to the superfamily of G-protein-coupled receptor (GPCR) polytopic
membrane-spanning proteins, being most closely related to the Frizzled
family of Wnt receptors (Wodarz and Nusse 1998; Dann et al. 2001
|
|
|
- mutant clones in the posterior compartment or far from the A/P boundary
develop normally (Chen,
1996)
- Two activated Smo mutants, SmoM1 and SmoM2, were isolated from human
basal cell carcinomas and are resistant to Ptc1 inhibition.
- M1 represents a change of Arg562 to Gln in the cytoplasmic tail
and was 50% inhibited by Ptc1 coexpression.
- SmoM1 showed a distribution that appeared to be a combination
of the wtSmo and SmoM2 patterns,with SmoM1+ juxtanuclear
structures superretained imposed on the ER pattern. (Incardona,
2002)
- M2 represents a change of Trp535 to Leu in the seventh transmembrane
segment and was uninhibited by Ptc1.
- distributions: differes most significantly from wild-type
Smo, appearing in a pattern virtually identical to an ER marker
and absent from the juxtanuclear region, where most of the intracellular
wtSmo resides. (Incardona,
2002)
- Drug treatment of the SmoM1 and SmoM2 mutants:
- Brefeldin A blocks ER-to-Golgi transport: Treatment of Smo
mutants: SmoM2-no effect. SmoM1- intensification of the juxtanuclear
component wtSmo-had a small degree of increase in the juxtanuclear
component. (Incardona,
2002)
- 6-hr chloroquine treatment: wtSmo-in large, swollen vesicular
structures (Figure 4G). SmoM2-retained its ER distribution (Figure
4I). SmoM1-showed a distribution intermediate to wtSmo and SmoM2
(Figure 4H), with a smaller fraction appearing in swollen vesicles
in addition to a large ER component. (Incardona,
2002)
|
Overexpression
/ Ectopic expression |
- overexpression of Smo is not sufficient to activate
the pathway, casting
doubt on the proposed stoichiometric relationship
between Ptc and Smo (Alcedo.
2000; Denef,
2000; Ingham,
2000).
- Increasing levels of Smo (4x UAS-Smo and UAS-Smohigh
driven with MS1096) leads to activation of low (Iro)
and then intermediate (dpp & ptc) Hh responses
(Figure 2) (Hooper,
2003)
|
|
|
| {Reagents} |
|
|
|
|
|