Gene Silencing by RNAi
The genes in our genomes are usually considered "good for us" and therefore conserved and carefully expressed through our lifetimes and the lifetimes of future generations. Yet paradoxically, an ancient biochemical mechanism exists to specifically silence gene expression. This system relies upon RNA to guide the silencing. Why is there such a system and how does it work?
The answer lies in knowing that not all DNA in our genomes is "good for us". Transposons are elements in the genome that propagate in number if given the opportunity. At least 45% of the human genome is derived from transposons, and their jumping around the genome is a major cause of mutagenesis. Viruses share sequence features with transposons, but they propagate independent of the genome and they are transmitted from one organism to the next through infection.
Mechanism of RNAi
If unchecked, viruses and transposons are potentially lethal. When viruses and transposons propagate, they produce double-stranded RNA (dsRNA). This serves as a molecular cue for cells that propagation is occurring (Figure 2). The cell then dices the dsRNA into small interfering RNAs (siRNAs) using an ribonuclease called Dicer (Lee et al., 2004). The siRNAs bind to an Argonaute protein, members of whom are found throughout the eukaryote phylogenetic tree. Single stranded RNA can basepair to a complementary siRNA within Argonaute, and if this happens, then Argonaute will cleave the phosphodiester backbone of the RNA strand that is paired to the siRNA. Argonaute has an unusual catalytic site for RNA cleavage (Kim et al., 200). When the RNA strand is cleaved, it is released from Argonaute and destroyed. The siRNA is then able to guide Argonaute to a new target RNA to destroy. This sets up a cycle of pairing-cleavage-unpairing that makes Argonaute a multi-turnover enzyme, specifically programmed by the guide siRNA it is stably associated with. The fidelity of RNA:RNA basepairing means the chance of accidentally cleaving the wrong RNA is less than 10-9. Argonaute not only cleaves RNA from the original gene from which it was derived but also RNA from all gene copies with similar sequence.
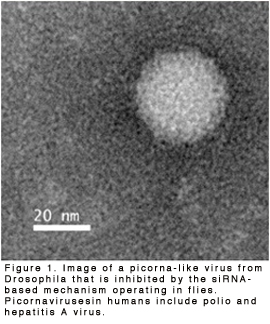
We have found that RNAi works as a powerful antiviral immune system, reducing both virus load and pathogenicity (Wang et al., 2006). and (Marques et al., 2013). Many animals and most plants rely on it as a major immune response to viral pathogens (Figure 1). We have also found the system works against transposons, reducing their expression and propagation (Marques et al., 2010).wo flavors of non-coding RNA carry out transposon silencing: siRNAs and piRNAs. The former primarily silences transposons in somatic cells of the body, and they also silence viruses. The latter RNA primarily silences transposons in the sperm and egg cells of the body. Special Argonautes collaborate with siRNAs and piRNAs(Pham et al., 2004 and Kennerdell et al., 2002).
Regulations of RNAi
The RNAi mechanism needs to work within the constraints of a cell, subject to changing conditions. Cells regulate RNAi to fit those conditions. One example is seen in tissue culture when cells are briefly starved of lipids and must adapt their metabolism (Figure 3). A kinase called PKC is shut off and this boosts the silencing strength of RNAi almost ten-fold. Argonautes get mobilized to silence gene expression at multiple levels, accounting for this super-charged silencing (Wu et al., Molecular Cell in press)If cells experience loss of insulin as well, this metabolic stress is met with more rapid turnover of Argonaute enzymes. Somehow the turnover is linked with an association of Argonaute with cytoplasmic membranous organelles such as endosomes(Wu et al., Molecular Cell in press).A similar relationship with turnover and endosomes is found in cells within an animal when endosomes traffic to lysosome-related organelles (Lee et al., 2009) and (Harris et al., 2011) .The reason why this relationship exists is tantalizingly mysterious but the fact that it is conserved from insects to humans argues that the reason is of general importance.
Engineered Silencing for Health and Biotechnology
If any dsRNA can associate with an Argonaute, it will silence its corresponding gene. The mechanism does not pick and choose according to whether a gene really is mobile or not. Therefore, we can program Argonautes according to our own purposes. This is done by synthesizing dsRNA and getting it into cells - once inside it associates with Argonaute and silences the targeted gene. This was first shown by Andrew Fire and Craig Mello for genes in the nematode Caenorhabditis elegans (Fire et al., 1998) and later by our group for genes in Drosophila (Kennerdell and Carthew., 1998).
|